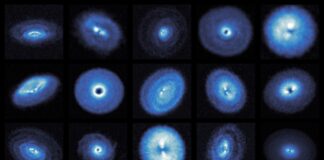
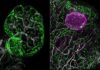
Researchers have developed a groundbreaking method called “zap-and-freeze” that allows scientists to observe brain cell activity with unprecedented detail. This technique involves rapidly stimulating neurons with electricity and then flash-freezing them within milliseconds, preserving their state for detailed study. The goal is to unlock insights into neurological conditions like Parkinson’s disease, where disrupted signaling plays a crucial role.
How ‘Zap-and-Freeze’ Works
The process combines electrical stimulation with ultra-fast cryogenic freezing under high pressure. This method captures brain cells in action, revealing dynamics that occur too quickly for conventional observation. The technique was tested on both mouse and human brain tissue by a team at Johns Hopkins University School of Medicine.
The results provided high-resolution details of synaptic function—the connections between neurons that handle communication—as well as the behavior of vesicles, which carry chemical messages between cells. These interactions are fundamental to cognitive processes like learning and memory.
Key Findings: Ultrafast Endocytosis
One critical discovery was the observation of ultrafast endocytosis, a recycling process where used vesicles are removed and new ones are created in under 100 milliseconds. This process is essential for maintaining continuous neuronal communication. The researchers also identified dynamin1xA as a key protein driving this endocytosis.
“This approach has the potential to reveal dynamic, high-resolution information about synaptic membrane trafficking in intact human brain slices,” explain the researchers in their published work.
The fact that these findings were consistent between mouse and human tissue supports the use of animal models in brain research, providing confidence in extrapolating results.
Implications for Parkinson’s Disease
Understanding how synapses and vesicles function at this granular level could be crucial for unraveling the mechanisms behind Parkinson’s disease. Neuronal death in Parkinson’s is thought to be linked to synaptic dysfunction. While the disease is complex, this technique could help pinpoint exactly what goes wrong at the molecular level.
The research team plans to extend their work by analyzing tissue samples from Parkinson’s patients undergoing brain surgery, comparing vesicle activity between healthy and affected brains. This could reveal specific differences that drive the progression of the disease.
Why This Matters
Parkinson’s disease affects millions worldwide, and its prevalence is expected to rise. Techniques like “zap-and-freeze” offer a vital tool for mapping brain activity at the smallest scales and shortest timeframes, potentially leading to more effective treatments. By visualizing synaptic membrane dynamics in live tissue, scientists can better understand both genetic and non-genetic forms of the condition.
The development of this method is a significant step forward in neuroscience, promising new avenues for studying brain function and treating neurological disorders.